バイオアベイラビリティーエンハンスメントスイート
Our Capabilities
カプスゲル Dosage Form Solutions has extensive capability, intellectual property and experience with the key technologies used to address bioavailability challenges:
- High-energy crystals / salt forms
- Solid amorphous dispersions
- Spray-dried dispersions (SDDs)
- ホットメルト押出 (Hot-Melt Extrusion、HME)
- Lipid- / Liquid-based formulation (LBF) approaches
- Nanocrystal technologies
- Specialized spray-dried nanoadsorbates (SDNAs)
- ナノ結晶固体分散体 (Solid NanoCrystalline Dispersions、SNCD)
- Specialized lipid multiparticulate (LMP) technology, used to combine bioavailability enhancement with extended release and / or taste-masking
This technology breadth allows our scientists to efficiently select technology, demonstrate formulation feasibility, optimize formulations and advance product concepts through clinical trials to ongoing commercial manufacture. Using our collective expertise in formulation science, we focus on key physical and chemical properties of the compound and dosage-form requirements, working collaboratively with clients to develop tailored problem statements specific to each compound. These problem statements are used to guide preliminary decisions about selection of the technology (i.e., drug-delivery platform) that offers the highest potential for performance, stability and manufacturability.
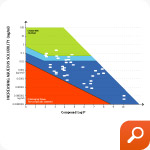 Our Technology Selection Methodology – developed over years of scientific investigation leveraging the experience of formulating and advancing more than 1,000 compounds – allows カプスゲル Dosage Form Solutions Scientists to select the optimal technology rapidly, saving valuable time, reducing complexity and lowering costs. Models and conceptual reference maps have been developed that guide scientists in the design process.
Our Technology Selection Methodology – developed over years of scientific investigation leveraging the experience of formulating and advancing more than 1,000 compounds – allows カプスゲル Dosage Form Solutions Scientists to select the optimal technology rapidly, saving valuable time, reducing complexity and lowering costs. Models and conceptual reference maps have been developed that guide scientists in the design process.
During feasibility analyses, our Rapid Advancement Process™ leverages these models to conduct feasibility studies in less than 6 weeks — and often and as little as 2 weeks. Our ability to formulate with minimal quantities of API means we can assist with lead selection by choosing the best compounds to progress at an early stage. First-in-human (FIH) clinical trials can be reached in 6 months or less from project initiation.
Formulation Development – Low-Solubility Compound Example
カプスゲル Dosage Form Solutions utilizes a wide range of complementary technologies to optimize finished dosage forms, including:
- modified-release technology suite,
- liquid and semi-solid fill technologies for high-potency API or other challenging compounds,
- multiparticulate formulation options,
- abuse-deterrent formulation approaches,
- inhalation formulation technologies, and
- biotherapeutic processing
Salt Form Selection and Polymorph Screening
カプスゲル Dosage Form Solutions offers preformulation services including polymorph identification and salt screen/selection as a complement to our Bioavailability Enhancement suite of technologies. Salt selection for a potential drug candidate can improve the bioavailability, stability and manufacturability of the drug. Considerations include crystalline material, non-hydroscopic, a high single melting temperature and chemical stability in the solid form. Salt forms of a drug have a large effect on the drugs’ quality, safety and performance.
Polymorph screening is done during API selection to determine if the API can exist in polymorphic forms and whether the choice of polymorph will affect the drug’s efficacy and/or safety profile. A polymorph screen of the drug substance is conducted utilizing a selection of possible solvents that have come in contact with or may come in contact with the drug substance during synthesis, purification, process, cleaning, and/or salt formation.