First Order Release - Lipid Multiparticulate (LMP) Technology
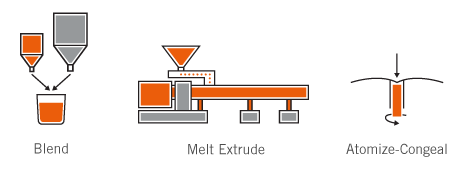
 Unique and proprietary melt-spray-congeal (MSC) technology is utilized to produce lipid multiparticulates with improved bioavailability and/or controlled release functionality.
Unique and proprietary melt-spray-congeal (MSC) technology is utilized to produce lipid multiparticulates with improved bioavailability and/or controlled release functionality.
Improved drug bioavailability is achieved through lipidic mechanisms while drug release modifications are achieved primarily through erosive mechanisms. A range of excipients and combinations is used to tailor functionality.
- Spherical, smooth 50-300 µm particles, typically with embedded drug substance, produced by a continuous spinning-disk process, amenable to fluid- bed coating for ultimate release control
- Benefits of multiparticulate format, e.g. improved gut distribution
- Good flow properties
- High drug load capability
- Taste-mask benefit
The drug physical form is dependent upon several factors including drug-excipient affinity, drug loading, drug melting temperature and process conditions.
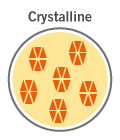 Crystalline drug formats facilitate good drug chemical stability and high drug loading, and yield immediate and controlled release functionality for even hydrophilic drugs.
Crystalline drug formats facilitate good drug chemical stability and high drug loading, and yield immediate and controlled release functionality for even hydrophilic drugs.
 Solubilized drug formats facilitate high loading for lipophilic drugs, improves bioavailability when the drug crystal lattice is limiting solubility, and provides either immediate or controlled release dependent upon LMP design
Solubilized drug formats facilitate high loading for lipophilic drugs, improves bioavailability when the drug crystal lattice is limiting solubility, and provides either immediate or controlled release dependent upon LMP design
Market precedence for LMP technology utilizing the MSC process has been achieved.
Manufacturing capability for lab through clinical quantities is in place at our Bend Research facility (Bend, OR). Commercial scale MSC production infrastructure is currently being installed at our Greenwood, SC manufacturing facility.
A simple yet effective MSC process, which is solvent-free with optional surfactant inclusion, is utilized for LMP production.